organic chemistry - Mechanism of acid-catalyzed ring opening of a cyclopropane ring - Chemistry Stack Exchange

Calculated transition states for cyclopropane ring-opening and their... | Download Scientific Diagram
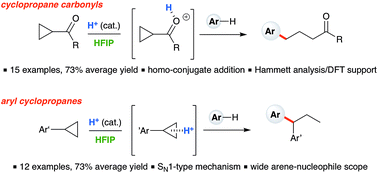
Ring-opening hydroarylation of monosubstituted cyclopropanes enabled by hexafluoroisopropanol - Chemical Science (RSC Publishing)
Catalysts | Free Full-Text | Divergent Reactivity of D-A Cyclopropanes under PTC Conditions, Ring-Opening vs. Decyanation Reaction
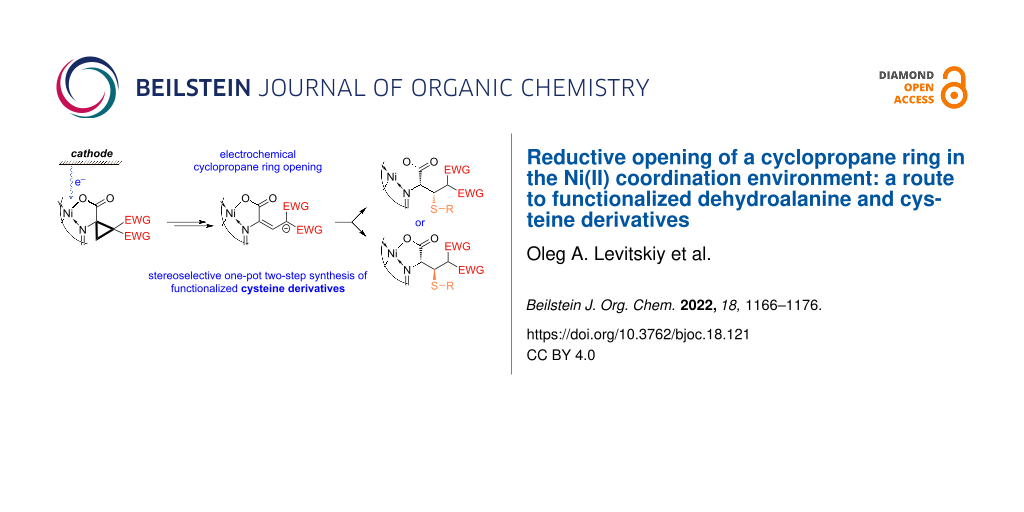
BJOC - Reductive opening of a cyclopropane ring in the Ni(II) coordination environment: a route to functionalized dehydroalanine and cysteine derivatives

Ring-opening hydroarylation of monosubstituted cyclopropanes enabled by hexafluoroisopropanol. - Abstract - Europe PMC
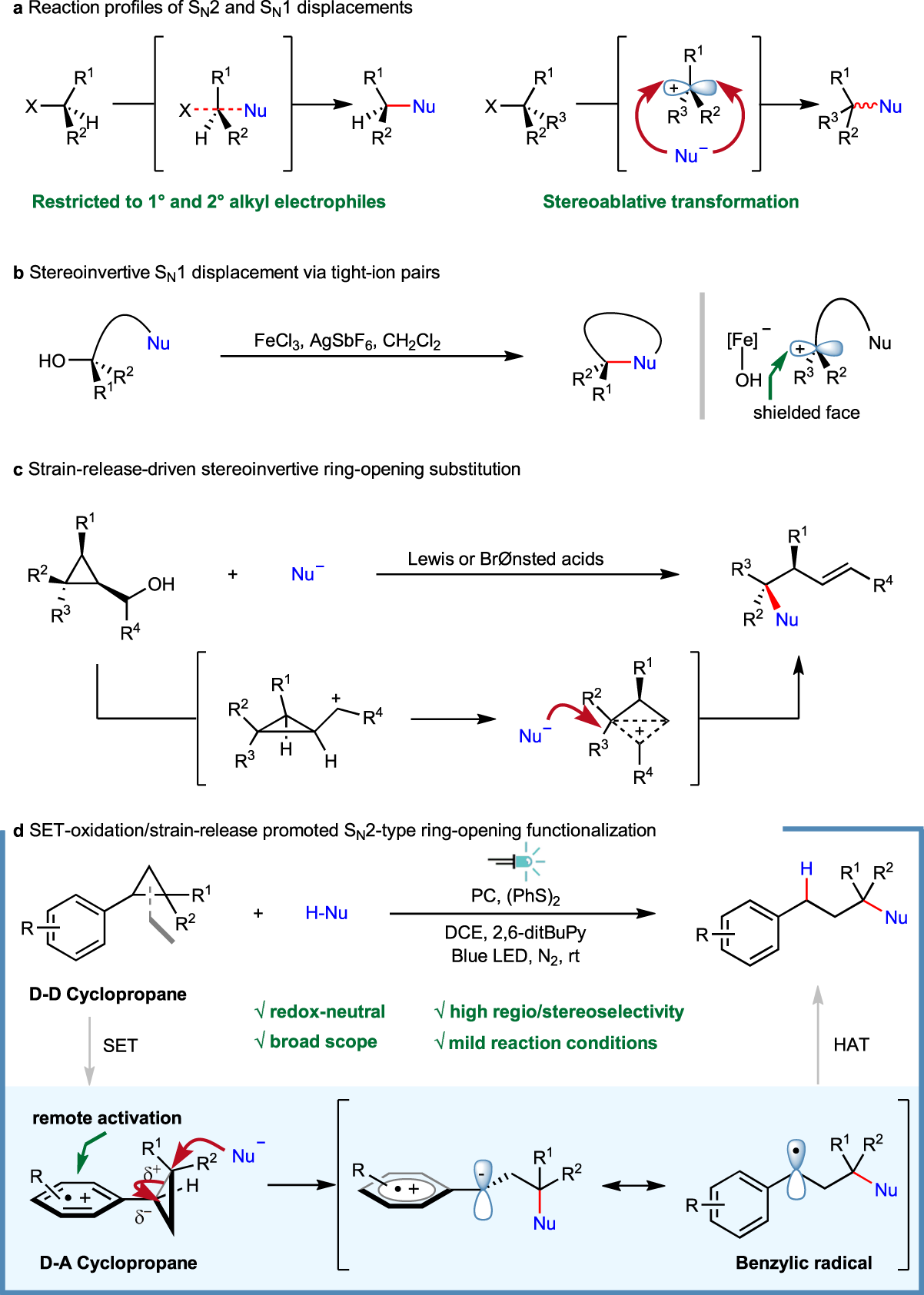
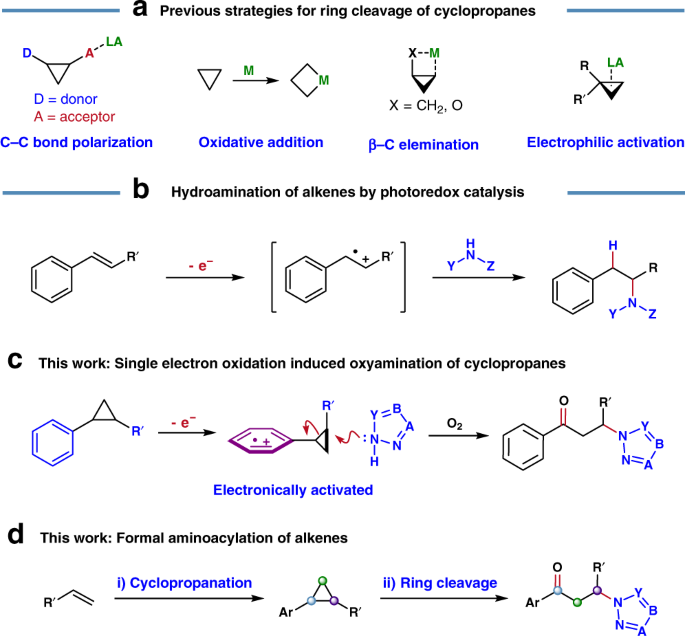
Photoredox-catalyzed C–C bond cleavage of cyclopropanes for the formation of C(sp3)–heteroatom bonds | Nature Communications

Visible‐Light‐Mediated Ring‐Opening Reactions of Cyclopropanes - Sivanandan - 2021 - European Journal of Organic Chemistry - Wiley Online Library
![Nucleophilic Ring Opening of Donor–Acceptor Cyclopropanes with the Cyanate Ion: Access to Spiro[pyrrolidone-3,3′-oxindoles] | The Journal of Organic Chemistry Nucleophilic Ring Opening of Donor–Acceptor Cyclopropanes with the Cyanate Ion: Access to Spiro[pyrrolidone-3,3′-oxindoles] | The Journal of Organic Chemistry](https://pubs.acs.org/cms/10.1021/acs.joc.8b00922/asset/images/acs.joc.8b00922.social.jpeg_v03)
Nucleophilic Ring Opening of Donor–Acceptor Cyclopropanes with the Cyanate Ion: Access to Spiro[pyrrolidone-3,3′-oxindoles] | The Journal of Organic Chemistry
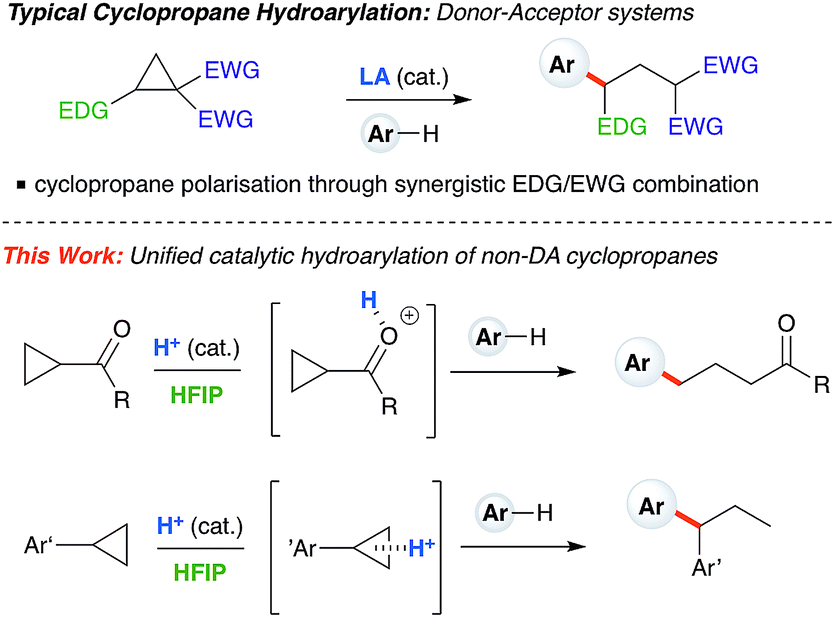
Ring-opening hydroarylation of monosubstituted cyclopropanes enabled by hexafluoroisopropanol - Chemical Science (RSC Publishing) DOI:10.1039/C8SC02126K

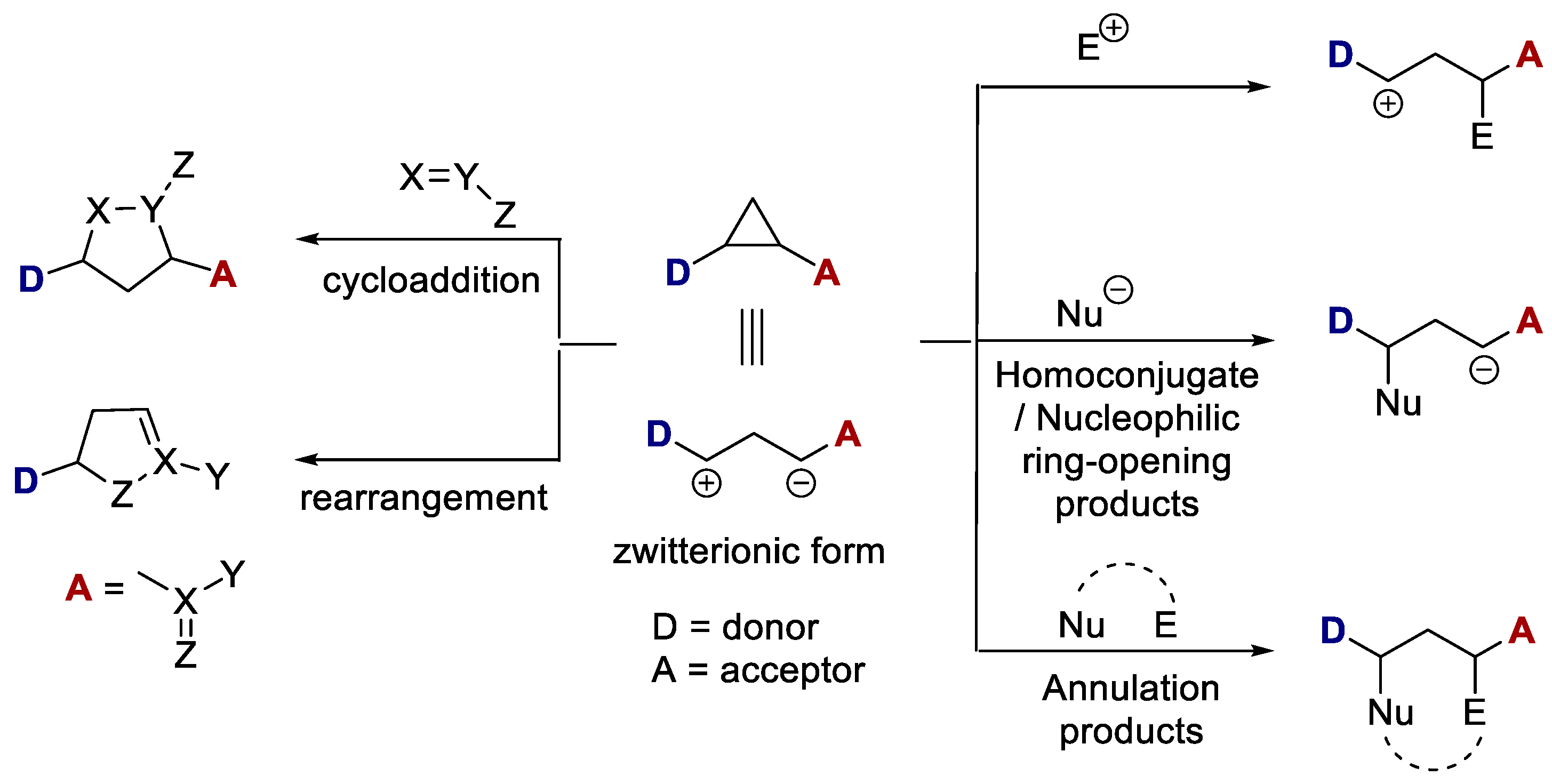
Recent Advances in the Chemistry of Doubly Activated Cyclopropanes: Synthesis and Reactivity | Bentham Science

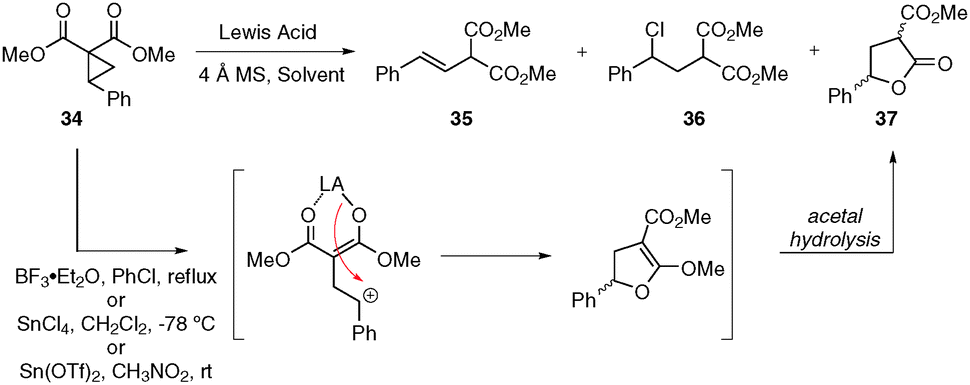
Calcium-Catalyzed, Dehydrative, Ring-Opening Cyclizations of Cyclopropyl Carbinols Derived from Donor-Acceptor Cyclopropanes. | Semantic Scholar

Nucleophilic Ring Opening of Donor–Acceptor Cyclopropanes Catalyzed by a Brønsted Acid in Hexafluoroisopropanol | Organic Letters

Nucleophilic ring opening of cyclopropane hemimalonates using internal Brønsted acid activation. | Semantic Scholar

organic chemistry - Mechanism of acid-catalyzed ring opening of a cyclopropane ring - Chemistry Stack Exchange
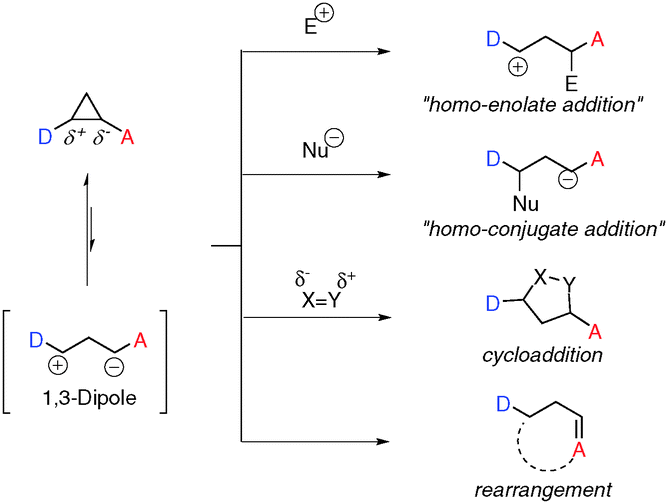
Intramolecular donor–acceptor cyclopropane ring-opening cyclizations - Chemical Society Reviews (RSC Publishing) DOI:10.1039/C3CS60238A






/chapter1/pages25and26/page25and26_files/cyclomech.png)